Background
In 2018, the Governance Committee for the implementation of the Government-wide Scientific Integrity PolicyFootnote 1 (the Governance Committee), whose membership includes the Chief Science Advisor, the Secretary of the Treasury Board (TB), and the President of the Professional Institute of the Public Service of Canada (PIPSC), published the Model Policy on Scientific Integrity (the model policy)Footnote 2. The model was drafted as a guide for 24 (now 25Footnote 3) federal departments and agencies that were required to implement a scientific integrity policy (SIP) under the terms of two collective agreements negotiated between PIPSC and TB in May and June of 2017. Since January 2020, implicated departments and agencies have been surveyed annually to determine their progress in implementing their scientific integrity policies and procedures. Results of the 2020 and 2021 surveys were reported in The Status of Federal Scientific Integrity Policies – February 2021Footnote 4 report, published in 2021. Results of the 2022 survey were reported in The Status of Federal Scientific Integrity Policies – April 2022Footnote 5. The current report summarizes results from the fourth annual survey conducted between December 2022 and late February 2023.
The 2023 survey
The 2023 survey requested information on 15 compliance measures, each of which is associated with a non-discretionary provision of the model policyFootnote 6. Since departments and agencies may adopt, adapt or even replace the model policy as they see fit, some compliance measures may not apply if, for example, a department or agency chose to eliminate the corresponding non-discretionary provision or made it discretionary in their policy.
The 2020 and 2021 surveys included thirteen compliance measures. For the 2022 survey, a fourteenth measure providing information on progress made in developing SIP performance monitoring and evaluation plans was added. In the 2023 survey, a fifteenth compliance measure providing information on measures in place to support training on evidence-informed decision-making was added.
The 2023 survey also included subsidiary questions associated with three original compliance measures. These subsidiary questions were designed to provide more information on employee notification about the departmental scientific integrity policy, Research Ethics Boards (REBs) and breach investigation procedures. The 2023 survey also included questions about potential amendments to the departmental policy and implementation actions taken by departments and agencies during the previous year.
As in the past, the 2023 report is based on a thorough review of all evidence provided by departments and agencies to support survey responses. Where the evidence provided was deemed insufficient to justify the submitted response, departments and agencies were contacted to discuss their responses and evidence. In some instances, these discussions resulted in revised survey responses.
2023 results
All 25 departments or agencies that are required to implement a scientific integrity policy responded to the survey. Twenty-four departments and agencies (representing 2,741 RE employees and 9,831 SP employeesFootnote 7, respectively) now have approved policies, 22 (representing 2,737 RE employees and 9,788 SP employees, respectively) of which are in effect (Fig.1).
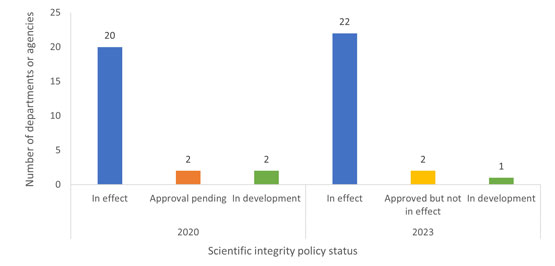
Figure 1
Fig. 1. The number of federal departments or agencies with scientific integrity policies in effect, awaiting approval, approved but not in effect, or in development (i.e., not yet submitted for approval) as of January 2020 and January 2023.
-
Figure 1 - Text version
The bar graph shows the number of federal departments or agencies (DDAAs) with scientific integrity policies in effect, awaiting approval, in development, or approved but still not in effect in 2020 and in 2023. For both years, the highest proportion is scientific integrity policies in effect, which rose from 20 in 2020 to 22 in 2023. DDAAs whose scientific integrity policies are awaiting approval decreased from 2 in 2020 to 0 in 2023, and those in development decreased from 2 in 2020 to 1 in 2023. There were no DDAAs whose scientific integrity policies were approved but not in effect in 2020, but in 2023 there are two DDAAs with policies approved by not in effect.
The model policy includes two key provisions in support of responsible conduct of research: peer review of all technical communications and, where required, approval of proposed research by a Research Ethics BoardFootnote 8. Eighteen of the 24 departments and agencies with approved SIPs have peer review requirements in place, while three departments and agencies reported that implementation of peer review requirements is still pending. Two departments and agencies elected not to report on the peer review compliance measure, and one departmentFootnote 9 considered the measure inapplicable.
Twelve departments and agencies have a process in place for ensuring that research proposals involving human subjects is reviewed by an REB, while three departments and agencies reported that implementation of an REB approval process is still pending. Two departments elected not to report on the REB compliance measure, and seven departments or agenciesFootnote 10 considered the measure to be inapplicable.
The model policy also includes non-discretionary provisions facilitating the federal science and research community in providing advice on regulations and policy, on departmental research programs, and on prioritizing investments in research.
- Seventeen of the 24 departments and agencies have processes in place for soliciting advice on policies and regulations, while five departments and agencies reported that implementation of these processes is still pending. For two departments this compliance measure is inapplicable as the corresponding provision was removed from their policy.
- Fifteen of the 24 have processes in place for soliciting advice on research programs and the prioritization of departmental research investment. Five departments and three departments reported that the implementation of processes for soliciting advice on research programs and the prioritization of departmental research investment, respectively, is still pending. For four departments, the implementation of processes for soliciting advice on research programs is inapplicable as the corresponding provision was removed from their policy. Similarly, for five departments, the implementation of processes for soliciting advice on the prioritization of departmental research investment is inapplicable as the corresponding provision was removed from their policy. One department decided not to report on the requirement for implementation of a process for soliciting researcher/scientist advice on prioritizing investments in research.
As of January 2023, 22 departments and agencies have notified their employees about the SIP, 12 of which regularly notify their employees about the policy. Two departments and agencies have yet to notify their employees about the SIP.
All 22 departments and agencies with SIPs in effect have appointed a Scientific Integrity Lead (SIL) who is responsible for overseeing allegations of scientific integrity breach. For at least two departments and agencies, the SIL is also responsible for promoting departmental scientific integrity. The two departments and agencies with approved SIPs that are not in effect have yet to appoint a SIL.
Eighteen departments and agencies have implemented a process for bringing forward allegations of scientific integrity breach. For 15 departments and agencies, this process is explicitly described in either a directive, policy, guidelines or guidance documents specific to alleged scientific integrity breaches or is part of a more general description that includes other types of alleged misconduct. For three departments and agencies, a process for bringing forward allegations of scientific integrity breach exists but is not explicitly described in a formal policy. Six departments and agencies have yet to implement a process for bringing forward allegations of scientific integrity breach.
The 2023 survey indicates that in the five years since the model policy was developed, departments and agencies have generally made good progress on implementation of their departmental SIPs (Fig 2). However, some significant gaps remain.
- Seven departments and agencies, have yet to implement measures to support education, training and/or professional development in responsible conduct in research; research ethics; and the annotation, management and archiving of research and scientific data.
- Ten departments and agencies have yet to implement processes for notifying external collaborators and contractors about SIPs.
- Eighteen departments have yet to implement measures to support education, training and/or professional development devoted to the roles of science and research in developing evidence to support evidence-informed decision-making.
- Most significantly, twenty-one departments or agencies have yet to implement a monitoring plan that provides information on the extent to which their policy has achieved its objectives (Figs 2, 3).
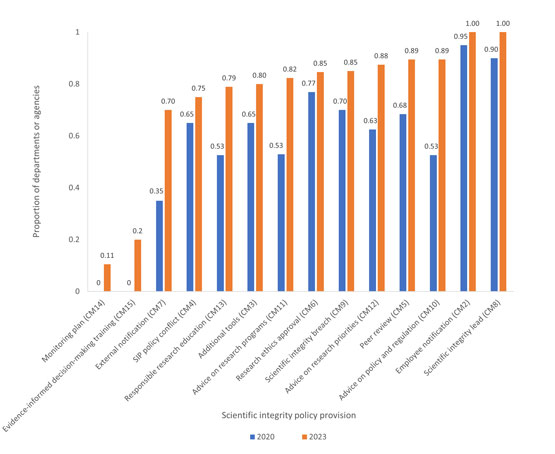
Figure 2
Fig. 2. The proportion of departments or agencies for which the actions corresponding to a specific provision in the model policy had been implemented by January 2020 or January 2023. This analysis includes only the 20 departments or agencies that had a SIP in effect in 2020. For each compliance measure (CM), departments or agencies who reported that the measure was inapplicable or who declined to report on the measure in the 2020 survey are excluded from the analysisFootnote 11. The 2020 survey did not include any questions concerning the status of SIP monitoring plans (CM14) or training about evidence-informed decision-making (CM15). For a description of the compliance measures, see Appendix B.
-
Figure 2 - Text version
The bar graph shows the proportion of the 20 departments and agencies with scientific integrity policies in effect in 2020 and that have implemented various scientific integrity policy provisions, and how these proportion of departments and agencies who have implemented various measures have changed between 2020 and 2023. The provision on SIP monitoring plan is the lowest at 0% in 2020 and 11% in 2023, followed by evidence-informed decision-making with the second lowest at 0% in 2020 and 20% in 2023. Provisions on employee notification and on appointing a scientific integrity lead have the highest percentages in 2020, at 95% and 90% respectively, and both at 100% in 2023. . The largest growth in implementation was in advice on policy and regulation which grew from 53% to 89%. None of the proportions shrank between 2020 and 2023.
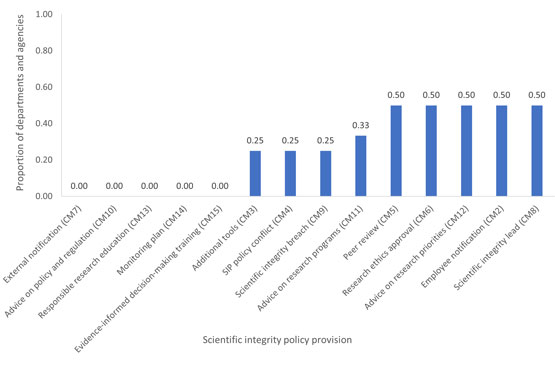
Figure 3
Fig. 3. The proportion of departments or agencies for which the actions corresponding to a specific provision in the model policy had been implemented by January 2023. This analysis includes only the four departments or agencies that had a SIP approved or come into effect after 2020. For each compliance measure (CM), departments who responded that the measure was inapplicable or who declined to report on the measure are excluded from the analysisFootnote 12. For a description of the compliance measures, see Appendix B.
-
Figure 3 - Text version
The bar graph shows the proportion of the 4 departments and agencies with scientific integrity policies approved and in effect after 2020 and that have implemented various scientific integrity policy provisions by January 2023. The provisions on peer review, research ethics approval, advice on research priorities, employee notification and on the appointment of scientific integrity lead are the highest, at 50%. The provisions on external notification, advice on policy and regulation, responsible research education, having a SIP monitoring plan and having training on evidence-informed decision-making are the lowest, at 0%.
During the past year, one department has amended its policy to clarify that their policy applies not only to RE and SP employees, but to any employee who designs, conduct, communicates, manages, reviews or make use of its research, science or related activities. Thirteen departments and agencies reported that they have produced additional procedures, policies, guidelines, tools, training, or professional development opportunities relevant to their policies. These include:
- Developing SIP and science ethics training materials.
- Developing and implementing policy or guidelines for a range of actions, including investigation of alleged breaches; external communications; and dissemination of research and scientific findings
- Creating internal and/or external webpages to provide information, tools, and resources for the departmental scientific integrity policy, including links to Government of Canada policies, the departmental scientific integrity policy, and SIP-related guidelines (such as guidelines for reporting and investigating alleged breaches of scientific integrity).
- Creating an action plan to strengthen the systematic integration of sex, gender, and diversity considerations into all of its research, regulations, programs and services.
- Investigating scientific integrity culture through the administering of employee surveys.
- Advancing mentoring programs in support of the implementation of the SIP; and
- Developing a process to notify/inform contractors or extramural collaborators of the SIP.
Over the past year, the Office of the Chief Science Advisor has continued to support federal departments and agencies in advancing federal scientific integrity. These initiatives included:
- In consultation with departments and agencies, publishing a SIP guidance document on peer review that includes some potential peer review processes that departments might adopt.
- Providing ongoing feedback to departments and agencies on their proposed SIP performance monitoring and evaluation strategies
- In collaboration with the Canada School for Public Service, producing a course on strengthening evidence-informed decision-making in government pursuant to the model policy s. 7.8Footnote 13; and
- Developing criteria to evaluate potential candidates for membership of committees investigating alleged breaches of scientific integrity.
The evolution of federal scientific integrity policies
We believe that the first (Version 1.0) model policy developed in 2017 and approved by the Governance Committee early in 2018, has helped to advance scientific integrity in federal departments and agencies. Of course, any definitive conclusions about just how effective it has been must wait until departments and agencies have evaluated the effects of policy implementationFootnote 14. But even in the absence of formal evaluation, there is a need to update the policy to reflect two realities:
- At the time of original drafting, several issues were identified as relevant to scientific integrity, but about which Version 1.0 is silent. At that time, it was considered that trying to address issues such as, for example, Indigenous Knowledge in the model policy was premature, and would need to wait until the federal government’s approach to (in this case) the issue of Indigenous Knowledge in the context of reconciliation was made clear.
- Since 2017, several important issues have come to the fore since the drafting of the first version of the policy that have clear implications to scientific integrity.
In addition, the original drafters recognized that at least some limitations of Version 1.0 would become evident only upon implementation. And indeed, the collective experience with implementation has identified several shortcomings of Version 1.0 that should be addressed.
For these three reasons, over the past year the Office of the Chief Science Advisor, in collaboration with PIPSC, federal departments and agencies, and the I-STEM cluster, has developed a first draft of an updated model policy which addresses:
- the appropriate solicitation, gathering, storage, communication, and use of Indigenous KnowledgeFootnote 15
- the resolution of potential tensions among open science, research security, and scientific integrity, and
- and the use of generative artificial intelligence tools for science and/or research activities.
Next steps
The 2022-2023 survey results demonstrates that considerable progress on scientific integrity has been made by federal departments and agencies. Building on this progress, over the next year, the Governance Committee will:
- Conduct consultations in order to update the Model Policy on Scientific Integrity
- Continue to provide ongoing advice on the development and implementation of departmental policy performance monitoring and evaluation plans; and
- Continue to monitor and evaluate SIP compliance and assist federal departments and agencies in resolving outstanding issues, especially those related to performance evaluation and education and training opportunities on topics related to scientific integrity.
As work continues, the Governance Committee is pleased with the progress made by federal departments and agencies on scientific integrity. The committee is keenly aware that this progress reflects the commitment of federal departments and agencies, Ministers, and employees to ensure that government decisions are informed by scientific evidence and that Canadians are informed about important scientific issues that affect them.
Appendix A: List of departments and agencies that are required to implement a scientific integrity policy
- Agriculture and Agri-Food Canada
- Canada Border Services Agency
- Canadian Food Inspection Agency
- Canadian Grain Commission
- Canadian Heritage
- Canadian Space Agency
- Correctional Service of Canada
- Crown-Indigenous Relations Northern Affairs Canada
- Department of National Defence
- Environment and Climate Change Canada
- Fisheries and Oceans Canada
- Global Affairs Canada
- Health Canada
- Impact Assessment Agency of Canada
- Indigenous Services Canada
- Infrastructure Canada
- Innovation, Science and Economic Development Canada
- Library and Archives Canada
- National Research Council
- Natural Resources Canada
- Public Health Agency of Canada
- Public Services and Procurement Canada
- Royal Canadian Mounted Police
- Statistics Canada
- Transport Canada
Appendix B: The scientific integrity policy (SIP) compliance survey questions
2021 SIP Compliance Survey Follow-Up Information:
- Did your department/agency complete the 2021 compliance survey?
- Have there been any amendments to your departmental scientific integrity policy (SIP) since the 2021 compliance survey?
- Has your department developed any additional procedures, policies, guidelines, tools, training or professional development opportunities relevant to the SIP since the 2021 survey?
Compliance Measures:
CM1. In what year did your departmental SIP come into effect?
CM2. (a) Has the department or agency notified employees about the SIP? (Corresponding mSIP article: s.7.1.1.1.)
(b) Does the department or agency regularly notify employees about the SIP?
CM3. Has the department or agency implemented additional procedures, policies, guidelines, tools, training or professional development opportunities in support of the SIP? (Corresponding mSIP article: s.7.1.2).
CM4. Does the department or agency have a process for reporting and recording instances of policy conflict or incompatibility with the SIP? (Corresponding mSIP article: s.3.5)
CM5. Does the department or agency require that all technical communications undergo peer-review? (Corresponding mSIP article: s.6.1, 7.5.6, and 7.8.1(i))
CM6. (a) Does the department or agency require that, where appropriate, research or scientific projects be reviewed and approved by a Research Ethics Board (REB)? (Corresponding mSIP article: s.6.1,7.5.6, and 7.8.1(i)) 7.8 (x)). See the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans – TCPS 2 (2018)
(b) Please identify the federal Research Ethic Boards (REBs) that your DDAA uses for project review and approval.
CM7. Has the department or agency taken steps to notify/inform contractors or extramural collaborators of the DDAA SIP and encourage them to comply with its articles? (Corresponding mSIP article: s.7.1.3)
CM8. Has the department or agency appointed a Science Integrity Lead? (Corresponding mSIP article: s.7.2.2.2)
CM9. (a) Does the department or agency have a process in effect for bringing forward and investigating breaches of scientific integrity? (Corresponding mSIP article: s.7.2.2.3)
(b) This process is explicitly described:
- a policy that applies specifically to alleged breaches of scientific integrity.
- a policy that applies to alleged breaches of scientific. Integrity as well as other types of misconduct.
- Other (please specify)
- Not applicable – this process is not explicitly described in a policy.
CM10. Does the department or agency have a mechanism and/or procedure in effect for soliciting researcher/scientist advice on departmental policies and/or regulations? (Corresponding mSIP article s.7.7.1)
CM11. Does the department or agency have a mechanism and/or procedure in effect for soliciting researcher/ scientist advice on departmental research programs? (Corresponding mSIP article s.7.7.2)
CM12. Does the department or agency have a mechanism and/or procedure in effect for systematically soliciting researcher/scientist assistance in identifying and prioritizing federal investment in research? (Corresponding mSIP article: s.7.7.3)
CM13. Does the department or agency have measures in effect to support education, training and/or professional development in any of the following areas: responsible conduct in research; research ethics; and the annotation, management and archiving of research and scientific data? (Corresponding mSIP article: s.7.2.1.3)
CM14. Does your department or agency have a monitoring plan in effect for the DDAA SIP that will provide information on the extent to which the policy has achieved its objectives (policy performance)?(Corresponding mSIP article: s.7.9)
CM15: Does your department or agency have measures in place to support education, training and/or professional development devoted to the roles of science and research in developing evidence to support evidence-informed decision-making. (Corresponding mSIP article: s.7.7.4)(Corresponding mSIP article: s.7.9)
